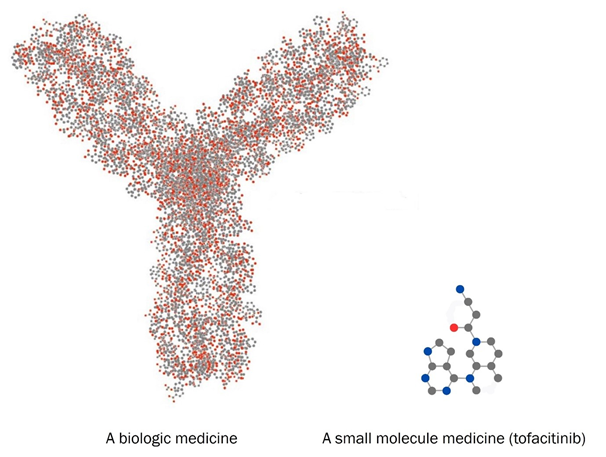
If the first biologic or targeted treatment you try is not right for you, you may need to change to a different one. The decision about which treatment to try next depends on several factors. Your IBD team should discuss this with you and make the decision together.
There are a few reasons you may need to change treatment.
Treatment does not work well
Biologics and other targeted medicines don’t work for everyone. Some people find that treatment does not help them feel better. Your IBD team will monitor how well your treatment is working.
If your symptoms do not improve within a few months, your IBD are likely to suggest stopping the medicine and trying a different option. Switching to a medicine that works in a different way could be an option.
Treatment stops working well
For some people, treatment works well at first but stops working over time.
For biologic medicines, this can happen because your immune system recognises the drug as a foreign substance and thinks it is harmful. It then produces proteins called antibodies against the biologic drug, which stop it working as well.
If this happens there may be a few options:
- Increase the dose
- Shorten the time between doses
- Add an immunosuppressant, if you are not already taking one, to help lower your antibody levels
- Change to a different medicine that works the same way
- Change to a medicine that works in a different way
Side effects are serious or hard to manage
Tell your IBD team if you are getting side effects. If you have side effects that are serious or hard to manage, they might suggest stopping treatment and trying a different option.
Washout period
When you change to a different medicine, your IBD team might recommend taking a break from treatment for a few weeks. This is to make sure all the first medicine is out of your system before you start the next one. It is called a washout period. The idea behind it is to keep your risk of infection as low as possible, but the evidence on whether or not it is needed is unclear.
A recent study found no difference in infection risk in people who had a washout period of less than 30 days compared to people who had a washout period of more than 30 days. So you might not need to stop treatment for long. Your IBD team will tell you what they recommend.